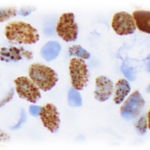
Exploratory clinical trials
We cover all aspect of Investigative Pathology from immunohistochemistry to digital Pathology and Molecular Biology and we can offer a complete package for your Clinical Trials

• Many validated biomarkers & Validation methods for new biomarkers
• Robust approaches to validating new markers
• Multiplexing panels already validated for immuno-oncology
• Robust technologies from Akoya & Roche
• Validation of each marker on each biopsy
• Identification of areas of interest and exclusion of necrotic parts…
• Confirmation of AI-based tissue segmentation
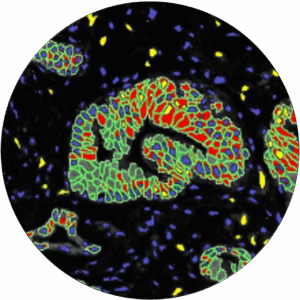
• High capacity bright-field and fluorescent slides scanners
• AI-based image analyses
• Spatial analyses and quantifications
• Patient slide anonymization in accordance with current regulatory standards.
• Slide batching by cohort or study criteria for optimal organization.
• Secure storage of digital slides on redundant servers (dual hosting).
• Secure sharing of digitized slides via a globally accessible platform.
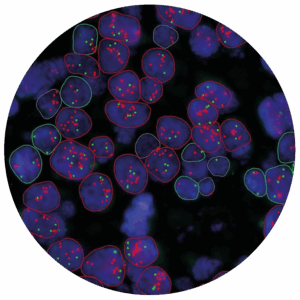
• Bright-field and fluorescence assays
• High-resolution slide scanning, Obj 60 (PathScan®)

• Extraction of DNA and RNA from paraffin tissue sections
• Purification and quality evaluation (nanodrop, Bioanalyzer; Q-bit…)
• Quantitative Q-PCR, digital PCR